Chapman - Figure 18 - Anti-oxidative activity Text
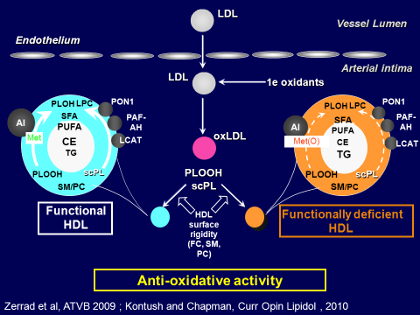
If we try to understand at the molecular level how all of these metabolic changes in HDL metabolism lead to defective function, we see in the Figure how HDL can be divided into functional HDL versus functionally deficient HDL.
On the left-hand side of the Figure, we see that in normal, functional HDL (typical of a healthy, normal lipidemic individual), the pro-inflammatory oxidized phospholipids, primarily hydroperoxides, are avidly picked up by HDL particles from LDL as LDL undergoes oxidation, and those oxidized phospholipids can be degraded either by paraoxonases or by Lp-PLA2 (or PAF acetylhydrolase as it is also called), or even by LCAP, because LCAP also exhibits antioxidative activity.
Those 3 enzymes can act together to protect against the deleterious effects of oxidized phospholipids. But equally, in the surface lipidome of normal functional HDL the sphingomyelin/phosphatidylcholine ratio is low, so that it can readily take up these oxidized phospholipids from LDL, and in that way, degrade them either by the action of those enzymes or, for example, by interaction of the hydroperoxides with apoA-I or apoM in such a way as to modify the methionine or tyrosine residues in those proteins.
Clearly, then, normal HDL particles can attenuate oxidative stress through several mechanisms that involve both the functions of the lipid components, the lipidome, as well as the protein components, the proteome.
Looking now at the right-hand side of the Figure there is functionally deficient HDL (typical, for example, of an individual with insulin resistance and cardiometabolic disease). In those individuals there are fewer HDL particles, at low concentrations, but equally important, the most recent analyses have shown that the particles are enriched in sphingomyelin and relatively poor in phosphatidylcholine. In insulin-resistant individuals that alteration results in greater rigidity in the surface of the HDL particles, with a reduced capacity to pick up the pro-inflammatory oxidized phospholipids from LDL.
Thus clearly a background of cardiometabolic disease with the different metabolic defects acts to change both the lipidome and also to some degree the proteome, through depletion of specific protective proteins such as ApoA-I and the various antioxidative enzymes. Acting together, factors in both the proteome and the lipidome are altered and this leads to functionally deficient HDL on a background of cardiometabolic disease.
J Clin Lipidol. 2011; 5(6).