Chapman - Figure 14 - Sphingosine and S1P Text
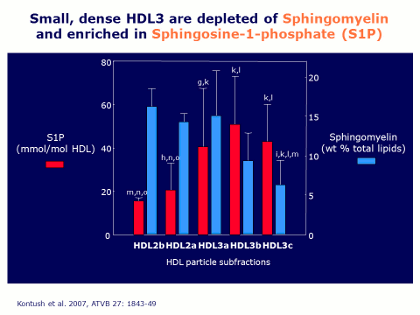
Now, looking in a little more detail at the lipidome of these different HDL subpopulations isolated from normal lipidemic healthy male subjects, the blue bars in the Figure represent sphingomyelin, and it is apparent that there is almost twice as much sphingomyelin in the large HDL particles (HDL2) compared with the sphingemyelin contents in the smaller HDL particles (HDL3).
This is of special interest because sphingomyelin is able to rigidify phospholipid structures; specifically, sphingomyelin can rigidify phosphatidylcholine molecules in a membrane or in the surface of the lipoprotein. Thus the implication is clear: the higher proportion of sphingomyelin in the large particles makes the surface rigid, whereas in the small particles the surface is much less rigid, or fluid – and this has been verified in experimental studies.
The fact that there is a low sphingomyelin content in the small HDL particles is also functionally important, because this means that the surface of those particles is more fluid, and this allows the small HDL3 particles to be very active acceptors of cholesterol and other lipids from plasma membranes of circulating cells, such as red blood cells, or from other lipoproteins. Again, a nice structural correlation with the functional observation that the small HDL2 particles are the ones that are very active in effluxing cholesterol. Or, in other words, the structural information we have obtained is entirely consistent with the functional information that we already possessed.
Looking again at the Figure, the green bars represent a second lipid, a minor lipid in HDL particles called sphingosine-1-phosphate (S1P). This is a very specialized lipid in its structure, and we know that it exerts a number of different cellular activities that promote an overall atheroprotective role for S1P. In addition, as shown by the green bars, S1P is preferentially transported by the small, dense HDL3 particles with the flexible and highly fluid surface, and that implies that that S1P in those small particles can be readily donated to different cell types when they are in the presence of HDL3. This, too, is highly relevant, because many cell types possess specific receptors for S1P, and the activation of those receptors exerts cytoprotective and cytoproliferative effects that we interpret at present to be primarily atheroprotective. As a result, once again there is a link between components of the lipidome of different HDL particles and some of the functions that we know HDL to possess.
J Clin Lipidol. 2011; 5(6).