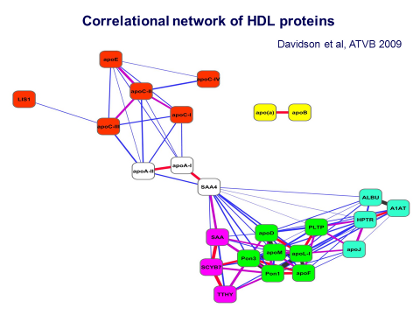
Chapman - Figure 10 - Correlational network Text
This Figure shows what is called a correlational network analysis. This analysis reveals that some of the proteins in the Class A pattern were tightly associated with each other, as shown by groupings of those proteins as a similar color. Thus looking at the 7 proteins in the green boxes — apoD, apoN, two paraoxonases (Pon1, Pon3), phospholipid transfer protein (PTLP), apoL-I, and apoF – reveals that these are very consistently and intimately associated within the HDL3 particles.
In addition there are the proteins in the turquoise boxes on the right and in the pinky-purple boxes on the left, showing other proteins that are also associated with HDL3, although perhaps in a less avid or less tight manner, such as the haptoglobin related protein (HPTR) or apoJ.
The Figure also shows that all of these green, turquoise, and pinky-purple proteins are in turn bound to the main proteins of HDL, ie, apoA-I and apoA-II, seen in the center. Thus all of these proteins seem to be associated in clusters on the densest and smallest subpopulation of HDL, the HDL3 particles.
The next question then was to ask, how might the characteristics of these proteins confer specialized functions on these small HDL3 particles? When we investigated this, we found that the group in green, all present in HDL3, were very highly correlated with the antioxidative activity of the HDL3 particles, and one of the reasons for that is purely chemical, ie, the paraoxonases can break down oxidized lipids, and apoN, for example, is a protein that contains amino acid residues such as methionine, which can be very readily attacked by oxidative or oxygen-free radicals.
Thus it can be seen that the HDL3 particles have several different mechanisms for attenuating oxidative stress and for inactivating oxygen-free radicals – which are, of course, abundant in cardiometabolic diseases such as diabetes.
It is also important to point out that apoA-I is also equipped to attenuate oxidative stress. As shown by Jay Heineke’s work from Seattle,[9] certain residues such as tyrosine and methionine appear to be preferential targets for oxygen-free radicals, and as a result apoA-I undergoes chemical modification with loss of part of its biological activities.
As a result of all these investigations, we start to understand how the clustering of these proteins in different HDL particle subpopulations relates to the specific biologic functions of these HDL particles.
J Clin Lipidol. 2011; 5(6).References
[9]For example: Heinecke J. The role of myeloperoxidase in HDL oxidation and atherogenesis. Curr Athero Rep 2007; 9(4); 249-251.