James A. Reiffel, MD - Novel Oral Anticoagulants - Figure 34
ROCKET AF: Design & ITT Analysis
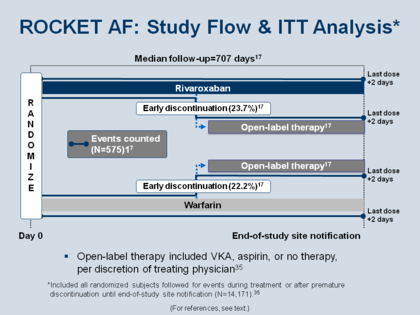
The Figure shows the study flow of ROCKET AF.[97][115] Patients received study drug from the time of randomization to the time of study site notification or until early discontinuation was deemed necessary, at which time patients were switched to open-label therapy. The trial was an intention-to-treat (ITT) design, which means that the outcomes in early discontinuation patients who went to open-label (approximately 23% in both arms) were included in the analysis of the results. In patients who started on one agent and then discontinued, the analysis was continued out until 2 days after the last dose (which will be important, as we will see in Figure 39).
Reiffel JA. Am J Med 2013; 126: 00-00.