James A. Reiffel, MD - Novel Oral Anticoagulants - Figure 17
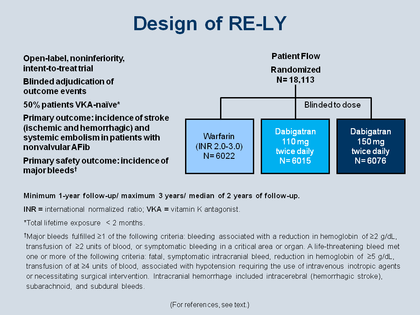
Design of RE-LY
The Figure shows the design of the RE-LY trial, the clinical trial that led to FDA approval for dabigatran.[96]
RE-LY was an open-label, non-inferiority, intention-to-treat trial of two different doses of dabigatran, 110 and 150 mg bid, versus warfarin. The physician and the patients were using the drugs unblinded, open-label (they knew whether the patient was getting warfarin or dabigatran), but they were blinded as to which dose of dabigatran dose if taking the trial drug. The adjudication committee, however, was blinded to all treatment, so the adjudication of events was done double-blind; in addition, 50% of the patients were vitamin K antagonist naïve. The primary outcome was incidence of systemic embolism or strokes of all types; the primary safety outcome was the incidence of major bleeding.
Reiffel JA. Am J Med 2013; 126: 00-00.