William French, MD - Anticoagulation: Special Populations - Figure 14
RE-LY, ARISTOTLE, and ROCKET AF
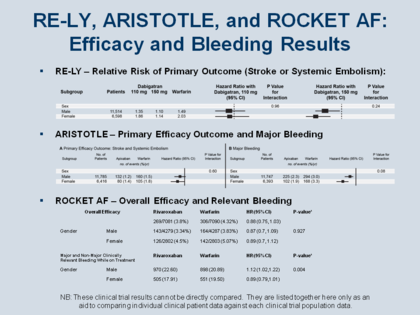
The results of the 3 large Phase 3 clinical trials of the 3 newly approved NOACs cannot be compared directly, as they represent different patient subsets, with different risk profiles and baseline characteristics, subjected to different therapeutic regimens, analyzed according to different statistical methods and criteria. However, it can be useful to congregate some of the principal efficacy and safety results in one convenient format as a way to facilitate aligning individual patient presentations with the individual clinical trial results.
As seen in the Figure, both men and women benefited from treatment in the first trial of the first NOAC to be approved, dabigatran, in the RE-LY trial,[129,130] with risk bleeding also superior to warfarin, although even more favorable in women. In ROCKET AF,[121] the benefit of prophylaxis with the factor Xa inhibitor rivaroxaban was statistically noninferior to the benefit with warfarin, but the risk of “major and non-major clinically relevant bleeding while on treatment” was greater than warfarin in men, but nonsignificantly better in women. In ARISTOTLE[125] with the second factor Xa inhibitor, apixaban, the benefit was more or less equal in terms of efficacy for men and women, but the bleeding was actually less in females.
French WJ. Am J Med 2013; 126: 00-00.
[RE-LY = Randomized Evaluation of Long-Term Anticoagulation Therapy]