James A. Reiffel, MD - Novel Oral Anticoagulants - Figure 11
Phase 3 Trials in Nonvalvular AFib
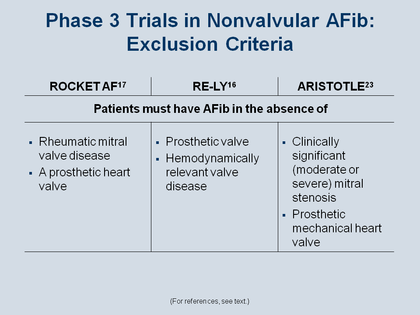
The Figure lists the definitions of “nonvalvular AFib” employed in these 3 pivotal trials – and it must be emphasized that
nonvalvular atrial fibrillation does not mean the patient has no valve disease
The definition of “nonvalvular atrial fibrillation” means that patients do not have a prosthetic heart valve that mandates anti-coagulation. The other important consideration for inclusion in a trial of anticoagulation therapy is that the patient must not have such severe heart disease that surgical intervention is required in the next year – because that would make it hard to enroll that patient in an anticoagulation trial. More specifically, the exclusion criteria for ARISTOTLE were clinically significant (moderate or severe) mitral stenosis, or a prosthetic mechanical heart valve [103] in ROCKET AF [97] and RE-LY[96] the exclusions were again hemodynamically relevant disease, prosthetic valves, or rheumatic valvular disease. So in all of these NOAC trials, patient with mitral prolapse or modest aortic stenosis or insufficiency were eligible for enrollment; in other words, these conditions are not considered “valvular heart disease” for this purpose.
Reiffel JA. Am J Med 2013; 126: 00-00.