Jin - Figure 37 - HPV Testing: Indications & FDA-Approved Tests Text
Jin - Figure 37 - HPV Testing: Indications & FDA-Approved Tests
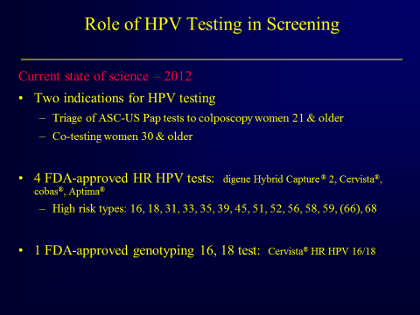
So, what is the current state of science in 2012 for HPV DNA testing? There are really two clinical indications
for this test:
- Triage women age ≥21 who have an ASC-US result by cytology to a high risk HPV DNA test. If that HR
HPV test is positive, she should go to colposcopy. - Cytology plus HPV co-testing for primary screening in women age ≥ 30.
Currently there are 4 FDA-approved HPV tests: digene Hybrid Capture® 2, Cervista®, cobas®, and Aptima®.
(There is also an FDA-approved genotyping test, Cervista® HPV16/18).